We have a patient with a venous ulcer, for severeal months, which has been stagnant for two weeks. Exudate has increased despite adequate compressive therapy and it is viscous, greenish and has a worse odor than in previous dressing changes. Wound edges are erythematous and warm. The patient reports more pain than usual and has a distermal sensation, without any other associated systemic symptoms. These clinical signs and symptoms suggest infection, so we will take a sample for microbiological culture.
Watch out! The microbiological study helps us identify the bacteria involved, but the diagnosis of infection is clinical. Therefore, the order of action will always be as follows: 1st suspicion of infection during the clinical examination, 2nd obtaining a sample for culture. In summary, this seems very simple, but these two points are a source of controversy, not to mention the selection of treatment, both topical and systemic!
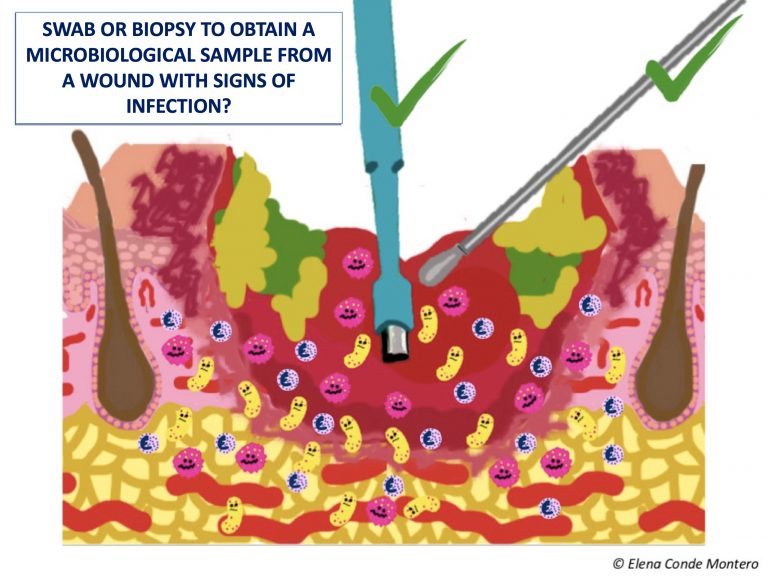
In this post we are going to focus on the eternal doubt when selecting the way to collect the sample: swab or punch biopsy?
I’m going to focus on leg wounds, which are the tyoe of wound that we treat most often in the clinical practice. In my clinic, in the presence of infection criteria in a leg ulcer, we usually collect the sample with a swab. However, in the absence of response to the antibiotic prescribed by the antibiogram, we perform a biopsy. In addition, if the torpid evolution of a leg ulcer makes us reconsider its etiology and we will perform a biopsy for histological study, if it meets the criteria for infection, we also send a tissue sample for microbiological study. In case of suspected infection by fungi or mycobacteria, we make sure that the biopsy is processed properly.
Now we will see what the studies and guidelines say, but first I will point out some general principles and summarize the characteristics of each technique.
General principles of collecting samples for culture:
- In a chronic wound with no signs of infection, bacterial contamination and colonising bacteria are found, which normally are not preventing healing. Therefore, since these bacteria do not require treatment, they do not need to be routinely sampled for identification.
- Although surface samples may not be an accurate reflection of the microbiological environment of the tissue at depth, any bacteria present in deep layers are very likely to be at the surface as well.1
- The amount of bacteria detected in a quantitative analysis of the sample cannot be considered as an isolated criterion for infection, since its clinical impact depends on the bacterial species and the patient’s immune response.
- Before sampling, the wound should be cleaned with saline and any devitalised tissue removed.
- In addition to wound swab and biopsy, there are other techniques, such as needle aspiration of the wound edge.
- The most frequently isolated bacteria in leg wounds with suspected infection is aureus, but it is usually polymicrobial flora.
- A proper protocol for processing the sample must be followed.
- Each laboratory will choose the microbiological techniques to be applied to each sample according to its availability.
Wound swab:
- This is the most practical and simple alternative. Some experts consider that, as it is a superficial sample, it can make it difficult to differentiate between contamination and infection.
- There are different techniques for taking the sample, but the most classic are the Levine technique (pressure in a 1 cm2 area of the wound bed for 5 seconds) and the zig-zag technique (displacement with rotation of the swab by 10 points through the entire wound bed, without touching slough or the edges of the lesion). Levine technique has shown greater reliability, having greater microbiological concordance with biopsy samples.2
- It allows a semi-quantitative study, which is easier to perform than quantitative studies
Punch biopsy:
- Tissue biopsy is a deep sample and it is the gold standard, that is, it is considered the most sensitive and specific test to detect the bacteria involved in the infection. However, its obtaining requires local anesthesia and, as it is an invasive procedure, it is not possible in all clinical settings.
- A fragment of tissue is removed from the wound bed, avoiding sloughy and necrotic tissue.
- The punch is a tool that facilitates its collection (usually we use 4 mm), but you can use a scalpel blade or a curette.
- After it has been carried out, the placement of alginate and pressure at the point where the sample has been taken promote haemostasia.

What do the studies say?
Studies are scarce, with few patients, and most of them do not specify the swabbing technique used. The correlation between the two techniques varies from non-existent to high. It is difficult to compare the results of studies with wounds of different aetiologies (diabetic foot, pressure ulcers, burns),3 so I will focus on leg wounds. I will highlight the study with the most favourable results for swabbing: “No more need for biopsies”.4 It included 46 patients with hard-to -heal venous ulcers. All wounds were cleaned with serum but not debrided. When comparing the bacterial species isolated with biopsy (punch 4 mm) and with swab (no method of collection specified), no significant differences were found. In order of prevalence, the most frequently isolated species in this Danish study were S. Aureus, E. Faecalis and Ps. Aeruginosa. This study also finds that the greater the number of samples collected, regardless of the technique, the greater the probability of finding a greater number of species. This is explained by the polymicrobial environment of chronic wounds, with a specific distribution of bacteria in the wound. Wound swabs, which may contact a larger area of the wound than the biopsy, might better reflect the microbiological environment of the wound. The authors conclude that the swab is an interesting technique for the microbiological study of infected wounds in our usual clinical practice and, after good cleansing and debridement, the superficial bacterial load corresponds to that in deep tissues.
A recent review5 on samples for culture in infected wounds highlights that, although the swab is a good option in the initial evaluation, in case antibiotic treatment does not obtain a clinical response, the biopsy test is interesting.
A conclusión that is repeated in the studies is that the microbiological result depends not only on the method used to collect the sample, but also on its adequate processing, including the type of swab material, the transport medium, the time to analysis and the interpretation criteria (qualitative, semi-quantitative or quantitative). The review “Microbiological diagnosis of skin and soft tissue infections “1 clearly explains the phases of sample collection, processing and interpretation.
What do the guidelines say?
To answer this question I have turned to the document published by the EWMA (European Wound Management Association) in 2016 “Management of patients with venous leg ulcers. Challenges and current best practice”, which reviews the recommendations of 8 clinical guidelines for the management of venous ulcers. The document emphasizes that diagnosis of infection is clinical, that all chronic wounds are colonized by bacteria and that sampling for culture is only of interest if there are signs and symptoms of infection. The recommendations on the technique for obtaining the sample include swab and biopsy, without detailing or mentioning the superiority of either.
When reviewing practical guidelines, with summary recommendations on how to perform the sample collection (such as this British example: Venous leg ulcers: Infection diagnosis and microbiology investigation), swab collection is the most widespread.
Following the recommendations of the SEIMC (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica), we should take more than one sample from different areas of the wound, since an isolated sample may not detect all the microorganisms causing the infection.
What do you do in your daily clinical practice?
References: